Today is World Oceans Day; a time to highlight human impact on the health of our oceans. In recent years, we have become increasingly aware of the amount of carbon dioxide (CO2) that human activity is releasing into the Earth’s atmosphere. What is less well known, is that one third of anthropogenic carbon emissions are absorbed from the atmosphere by Earth’s oceans. While this helps to reduce the amount of CO2 that stays in the atmosphere, it also has an impact on ocean chemistry. The increased amount of CO2 in seawater lowers the pH of the ocean, making it more acidic. This process is known as ocean acidification and disrupts the marine carbonate system.
It’s currently not possible to directly measure pH from space, and proxies like temperature, salinity, and biological activity are used to infer changes in acidity. During my Fellowship placement in the Ocean Biogeochemistry (OceanBug) group at the University of Oxford, I am using the experience I gained during my PhD looking for subtle organic signatures on Mars to find ways to directly detect acid in seawater. Using laboratory analogue experiments, as I did for Martian studies, we hope to establish detectability limits for boric acid in seawater samples, to combine in-situ pH measurements with satellite data to interpret historical trends and develop new ways of monitoring ocean acidification from space to better inform scientists, policy makers and the public about how we can protect our oceans.
Increased acidity of seawater affects coral reefs and other marine life health, as these changes in ocean chemistry prevent corals and other organisms from forming their shells and extensive skeletons. In turn, this has a massive impact on marine biodiversity, as coral reefs are thought to be one of the most diverse ecosystems on the Earth, accounting for around 25% of marine life while occupying less than 1% of the ocean floor surface area. Measuring ocean acidification is crucial to understanding what has happened in the past and predicting future ocean chemistry conditions. Understanding trends in ocean acidity will enable the development and provision of strategies to protect marine life and, more broadly, preserve the buffering role of the oceans to mitigate the effects of anthropogenic climate change.
By using interdisciplinary approaches to science, we can utilize remote sensing and laboratory analogue methods that are so commonly used when studying other planets to get a better understanding of our own. – Dr. Jacqueline Campbell
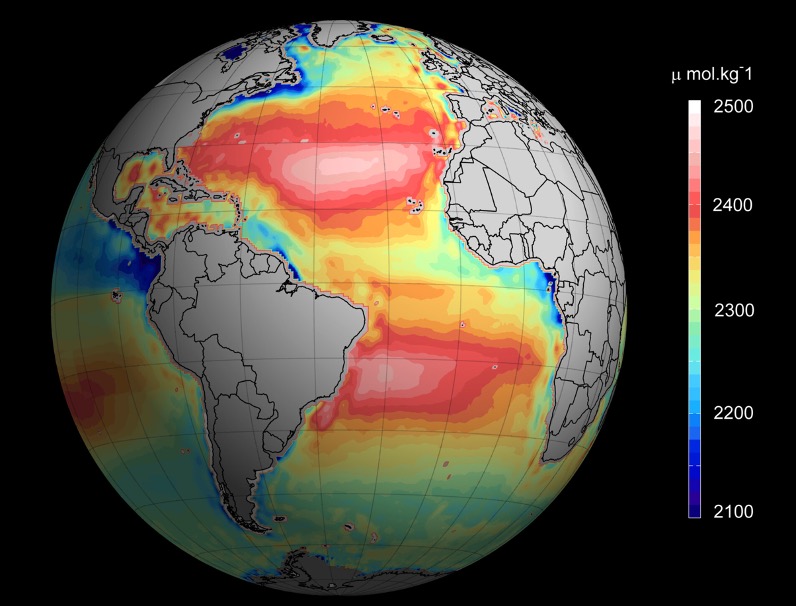
Historically, ocean acidification is tracked by measuring the pH of seawater samples taken from static monitoring stations or by scientific vessels. While this is in excellent way to get very accurate pH readings, it means your measurements are limited to specific sites for monitoring stations, and to short periods of time for sea vessels. To get a global understanding of the problem, satellite remote sensors are a very useful tool as they can record conditions anywhere on Earth over many years.
By using interdisciplinary approaches to science, we can utilize remote sensing and laboratory analogue methods that are so commonly used when studying other planets to get a better understanding of our own. Transcending disciplines and removing boundaries between scientific fields is vital to advancing our knowledge of complex problems like climate change. It is my hope that what I have learned about in how to study the dry landscapes of Mars will give us a better understanding of the Earth’s vast marine environments as we celebrate World Oceans Day.